Benzimidazole-loaded Halloysite Nanotube as a Smart Coating Application
Keywords:
Benzimidazole, Halloysite nanotube, Nanocontainer, Smart coatingAbstract
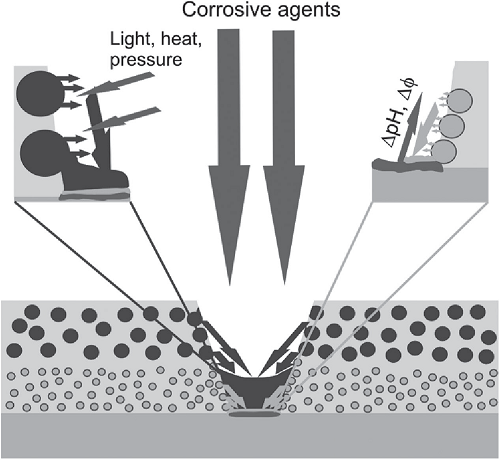
Smart coating has been developed for the corrosion control of surfaces exposed to corrosive environment. An important step in development of a smart coating is the successful impregnation of corrosion inhibitor into the nanocontainer as a coating pigment. In this study, halloysite was used as nanocontainer to encapsulate benzimidazole as corrosion inhibitor by vacuum method. FESEM, TEM, FTIR and TGA characterization techniques were used to confirm the loading of halloysite with benzimidazole. FESEM results indicated differences between the morphology of the unloaded-halloysite and benzimidazole loaded-halloysite. TEM results confirmed that benzimidazole molecules are loaded into halloysite. FTIR result revealed there are differences in the absorbance characteristic of peaks between peak number 1000-4000 cm-1 for loaded and unloaded samples. It is seen that the absorbance in the loaded-halloysite is higher than unloaded-halloysite, which confirms quantity/specific functional group of molecules. TGA result showed the temperature of degradation of benzimidazole-loaded HNT was higher than pure HNT. EIS was conducted to examine the protection characteristic of the developed smart coating. From EIS results, of 1, 3, and 6 days of experimental duration, it is seen that the value of coating impedance (Z’) after exposure to 3.5% NaCl environment is very height, 2.460E+07 Ω, which confirm a very good anti corrosion protection characteristic for the developed smart coating.
References
E. Abdullayev, V. Abbasov, A. Tursunbayeva, et al., “Self-healing coatings based on halloysite clay polymer composites for protection of copper alloys,” ACS Applied Materials and Interfaces, vol. 5, no. 10, pp. 4464-4471, April 2013.
A. Popoola, O. E. Olorunniwo, and O. O. Ige, “Corrosion resistance through the application of anti-corrosion coatings,” Developments in Corrosion Protection, vol. 2, no. 12, pp. 241-270, February 2014.
D. G. Shchukin, S. V. Lamaka, K. A. Yasakau, M. L. Zheludkevich, M. G. S. Ferreira, and H. Mohwald, “Active anticorrosion coatings with halloysite nanocontainers,” The Journal of Physical Chemistry C, vol. 112, no. 4, pp. 958-964, January 2008.
E. Abdullayev, R. Price, D. Shchukin, and Y. Lvov, “Halloysite tubes as nanocontainers for anticorrosion coating with benzotriazole,” ACS Applied Materials and Interfaces, vol. 1, no. 7, pp. 1437-1443, June 2009.
Y. M. Lvov, D. G. Shchukin, H. Mohwald, and R. R. Price, “Halloysite clay nanotubes for controlled release of protective agents,” ACS Applied Materials and Interfaces, vol. 2, no. 5, pp. 814-820, May 2008.
M. Liu, Z. Jia, D. Jia, and C. Zhou, “Recent advance in research on halloysite nanotubes-polymer nanocomposite,” Progress in Polymer Science, vol. 39, no. 8, pp. 1498-1525, August 2014.
E. Abdullayev and Y. Lvov, “Clay nanotubes for corrosion inhibitor encapsulation: release control with end stoppers,” Journal of Materials Chemistry, vol. 20, no. 32, p. 6681, August 2010.
A. Mobinikhaledi, N. Foroughifar, P. Mohammadlu, and M. Kalhor, “Synthesis of some benzimidazole-substituted benzotriazoles,” South African Journal of Chemistry, vol. 61, no. 1, pp. 141-143, 2008.
N. Vijayan, G. Bhagavannarayana, R. Ramesh Babu, R. Gopalakrishnan, K. K. Maurya, and P. Ramasamy, “A comparative study on solution- and bridgman-grown single crystals of benzimidazole by high-resolution x-ray diffractometry, fourier transform infrared, microhardness, laser damage threshold, and second-harmonic generation measurements,” Crystal Growth Design, vol. 6, no. 6, pp. 1542-2546, May 2006.
N. A. Abood and B. A. Saeed, “Structures and vibrational frequencies of imidazole, benzimidazole and its 2-alkyl derivatives determined by DFT calculations,” Basrah Journal of Science ( C ), vol. 30, no. 1, pp. 119-131, 2012.
M. F. Morks, P. Corrigan, N. Birbilis, and I. S. Cole, “A green MnMgZn phosphate coating for steel pipelines transporting CO2 rich fluids,” Surface and Coatings Technology, vol. 210, pp. 183-189, October 2012.
Zaki Ahmad, Principles of corrosion engineering and corrosion control. Elsevier Science & Technology Books, 2006.
Q. Bai and Y. Bai, Subsea pipeline design, 1st ed. Waltham: Elsevier, 2014, pp. 451-464.
D. Grigoriev, E. Shchukina, and D. G. Shchukin, “Nanocontainers for self-healing coatings,” Advanced Materials Interfaces, vol. 4, no. 1, June 2016.
R. Meirowitz, “Microencapsulation technology for coating and lamination of textiles,” Smart Textile Coatings and Laminates, vol. 3, no. 12, pp. 125-154, 2010.
M. Sauer, D. Streich, W. Meier, B. M. Sauer, D. Streich, and W. Meier, “pH-sensitive nanocontainers,” Advanced Materials, vol. 13, no. 21, pp. 1649-1651, November 2001.
S. Simoes, J. Nuno Moreira, C. Fonseca, N. Duzgunes, and M. C. Pedroso De Lima, “On the formulation of pH-sensitive liposomes with long circulation times,” Advanced Drug Delivery Reviews, vol. 56, no. 7, pp. 947-965, April 2004.
T. Y. Liu and Y. L. Lin, “Novel pH-sensitive chitosan-based hydrogel for encapsulating poorly water-soluble drugs,” Acta Biomaterialia, vol. 6, no. 4, pp. 1423-1429, April 2010.
J. Qian and C. Berkland, “pH-sensitive triblock copolymers for efficient siRNA encapsulation and delivery,” Polymer Chemistry, pp. 3472-3479, May 2015.
J. Gómez-estaca, R. Gavara, and P. Hernández-Muñoz, “Encapsulation of curcumin in electrosprayed gelatin microspheres enhances its bioaccessibility and widens its uses in food applications,” Innovative Food Science & Emerging Technologies, vol. 29, pp. 302-307, May 2015.
K. M. Rao, K. S. V. K. Rao, G. Ramanjaneyulu, and C. S. Ha, “Curcumin encapsulated pH sensitive gelatin based interpenetrating polymeric network nanogels for anti cancer drug delivery,” International Journal of Pharmaceutics, vol. 478, no. 2, pp. 788-795, January 2015.
T. C. Lai, J. Yu, and W. B. Tsai, “Gelatin methacrylate/carboxybetaine methacrylate hydrogels with tunable crosslinking for controlled drug release,” Journal of Materials Chemistry B, vol. 4, no. 13, pp. 2304-2313, April 2016.
Y. Guo et al., “RGD-decorated redox-responsive d-α-tocopherol polyethylene glycol succinate-poly(lactide) nanoparticles for targeted drug delivery,” Journal of Materials Chemistry B, vol. 4, no. 13, pp. 2338-2350, April 2016.
M. Liu et al., “Functionalized halloysite nanotube by chitosan grafting for drug delivery of curcumin to achieve enhanced anticancer efficacy,” Journal of Materials Chemistry B, vol. 4, no. 13, pp. 2253-2263, April 2016.
Y. Lv, L. Hao, W. Hu, Y. Ran, Y. Bai, and L. Zhang, “Novel multifunctional pH-sensitive nanoparticles loaded into microbubbles as drug delivery vehicles for enhanced tumor targeting,” Scientific reports, vol. 6, p. 29321, April 2016.
I. Hofmeister, K. Landfester, and A. Taden, “PH-Sensitive nanocapsules with barrier properties: Fragrance encapsulation and controlled release,” Macromolecules, vol. 47, no. 16, pp. 5768-5773, August 2014.
C. G. Dariva and A. F. Galio, “Corrosion Inhibitors - principles mechanisms and applications,” Developments in Corrosion Protection, p. 16, 2014.
D. Gopi, E. M. Sherif, V. Manivannan, D. Rajeswari, M. Surendiran, and L. Kavitha, “Corrosion and corrosion inhibition of mild steel in groundwater at different temperatures by newly synthesized benzotriazole and phosphono derivatives,” Industrial & Engineering Chemistry Research, vol. 53, no. 11, pp. 4286-4294, February 2014.
P. Yuan, D. Tan, and F. Annabi-Bergaya, “Properties and applications of halloysite nanotubes: recent research advances and future prospects,” Applied Clay Science, vol. 112-113, pp. 75-93, August 2015.
E. Joussein, S. Petit, J. Churchman, B. Theng, D. Righi, and B. Delvaux, “Halloysite clay minerals-a review,” Clay Minerals, vol. 40, no. 4, pp. 383-426, December 2005.
J. Zhang, J. Hu, J. Zhang, and C. Cao, “Studies of water transport behavior and impedance models of epoxy-coated metals in NaCl solution by EIS,” Progress in Organic Coatings, vol. 51, no. 2, pp. 145-151, November 2004.
S. Masadeh, “Electrochemical impedance spectroscopy of epoxy-coated steel exposed to dead sea water,” Journal of Minerals and Materials Characterization and Engineering, vol. 4, no. 2, pp. 75-84, October 2005.
M. Behzadnasab, S. M. Mirabedini, K. Kabiri, and S. Jamali, “Corrosion performance of epoxy coatings containing silane treated ZrO2 nanoparticles on mild steel in 3.5 % NaCl solution,” Corrosion Science, vol. 53, no. 1, pp. 89-98, January 2011.
Published
How to Cite
Issue
Section
License
Copyright (c) 2017 International Journal of Engineering and Technology Innovation

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright Notice
Submission of a manuscript implies: that the work described has not been published before that it is not under consideration for publication elsewhere; that if and when the manuscript is accepted for publication. Authors can retain copyright in their articles with no restrictions. Also, author can post the final, peer-reviewed manuscript version (postprint) to any repository or website.

Since Jan. 01, 2019, IJETI will publish new articles with Creative Commons Attribution Non-Commercial License, under Creative Commons Attribution Non-Commercial 4.0 International (CC BY-NC 4.0) License.
The Creative Commons Attribution Non-Commercial (CC-BY-NC) License permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.


.jpg)


