Evaluation of Activation Energy for Agricultural Residues with Ignition Temperature
DOI:
https://doi.org/10.46604/ijeti.2022.8889Keywords:
agricultural residues, ignition, combustion, activation energyAbstract
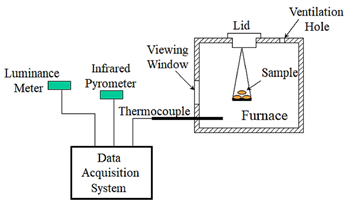
The objective of this study is to evaluate the activation energy of agricultural residues with their ignition characteristics. The ignition temperature of agricultural residues (peanut shell, rice hull, and rice straw) is determined by measuring particle temperature, particle luminosity, and gas temperature for samples weighing 2.0, 2.5, and 3.0 grams. The maximal slope of the particle temperature versus furnace temperature is used to determine the occurrence of ignition. Values of activation energy are analyzed by the Semenov model with the measured ignition temperature. Results show that the particle ignition temperature is 317, 324, and 330°C for rice straw, peanut shell, and rice hull, respectively. The results also indicate that the particle ignition temperature reduces as the volatile content increases and the sample amount decreases. The value of activation energy is 157.2, 170.3, and 192.8 kJ/mole for rice straw, peanut shell, and rice hull, respectively.
References
D. Magalhães, F. Kazanç, A. Ferreira, M. Rabaçal, and M. Costa, “Ignition Behavior of Turkish Biomass and Lignite Fuels at Low and High Heating Rates,” Fuel, vol. 207, pp. 154-164, November 2017.
J. L. Lin, H. M. Keener, and R. H. Essenhigh, “Pyrolysis and Combustion of Corncobs in a Fluidized Bed: Measurement and Analysis of Behavior,” Combustion and Flame, vol. 100, pp. 271-282, 1995.
J. B. Howard and R. H. Essenhigh, “Mechanism of Solid-Particle Combustion with Simultaneous Gas-Phase Volatile Combustion,” Symp. International on Combustion, vol. 11, no. 1, pp. 399-408, 1967.
R. H. Essenhigh, M. K. Misra, and D. W. Shaw, “Ignition of Coal Particles: A Review,” Combustion and Flame, vol. 77, no. 1, pp. 3-30, July 1989.
J. L. Lin, C. S. Lee, and C. T. Sheng, “Measurement and Analysis of Ignition Behavior for Peanut Shell during Combustion,” Journal of Agricultural Machinery, vol. 7, no. 3, pp. 61-74, September 1998.
J. M. Jones, A. Saddawi, B. Dooley, E. J. S. Mitchell, J. Werner, D. J. Waldron, et al., “Low Temperature Ignition of Biomass,” Fuel Processing Technology, vol. 134, pp. 372-377, June 2015.
H. Fatehi, W. Weng, M. Costa, Z. Li, M. Rabaçal, M. Aldén, et al., “Numerical Simulation of Ignition Mode and Ignition Delay Time of Pulverized Biomass Particles,” Combustion and Flame, vol. 206, pp. 400-410, August 2019.
F. Shan, Q. Lin, K. Zhou, Y. Wu, W. Fu, P. Zhang, et al., “An Experimental Study of Ignition and Combustion of Single Biomass Pellets in Air and Oxy-Fuel,” Fuel, vol. 188, pp. 277-284, January 2017.
P. E. Mason, L. I. Darvell, J. M. Jones, M. Pourkashanian, and A. Williams, “Single Particle Flame-Combustion Studies on Solid Biomass Fuels,” Fuel, vol. 151, pp. 21-30, July 2015.
H. J. Mühlen and F. Sowa, “Factors Influencing the Ignition of Coal Particles: Studies with a Pressurized Heated-Grid Apparatus,” Fuel, vol. 74, no. 11, pp. 1551-1554, 1995.
J. Pedro, S. Seixas, and R. H. Essenhigh, “Ignition Temperature of a High-Ash Portuguese Anthracite: Comparison with a Low-Ash Pennsylvania Anthracite,” Combustion and Flame, vol. 66, no. 2, pp. 215-218, November 1986.
T. F. Wall, R. P. Gupta, V. S. Gururajan, and D. K. Zhang, “The Ignition of Coal Particles,” Fuel, vol. 70, no. 9, pp. 1011-1016, September 1991.
Z. Zhang, M. Zhu, J. Li, K. Zhang, G. Xu, and D. Zhang, “Experimental Study of Ignition and Combustion Characteristics of Single Particles of Zhundong Lignite,” Energy and Fuels. vol. 32, no. 4, pp. 4221-4226, 2018.
C. O. Gomez and F. J. Vastola, “Ignition and Combustion of Single Coal and Char Particles: A Quantitative Differential Approach,” Fuel, vol. 64, no. 4, pp. 558-563, 1985.
R. P. Gupta, V. S. Gururajan, J. A. Lucas, and T. F. Wall, “Ignition Temperature of Pulverized Coal Particles: Experimental Techniques and Coal-Related Influences,” Combustion and Flame, vol. 79, no. 3-4, pp. 333-339, March 1990.
H. Karcz, W. Kordylewski, and W. Rybak. “Evaluation of Kinetic Parameters of Coal Ignition,” Fuel, vol. 59, no. 11, pp. 799-802, 1980.
P. G. Sweeny and D. T. Grow, “Ignition and Combustion Characteristics of a Lignite,” Energy and Fuels, vol. 3, no. 6, pp. 678-685, 1989.
Y. Zhou, X. Jin, and W. Chu, “Quantitative Measurement and Indication of Pulverized Coal Ignition Temperatures in O2/CO2 Environments,” Applied Thermal Engineering, vol. 112, pp. 888-894, 2017.
W. B. Fu, Y. Ge, M. H. Tang, and T. F. Liu, “A Study on Ignition Criterion of a Large Carbon/Char Particle,” Combustion Science and Technology, vol. 108, no. 1-3, pp. 91-101, 1995.
C. L. Sun and M. Y. Zhang, “Ignition of Coal Particles at High Pressure in a Thermogravimetric Analyzer,” Combustion and Flame, vol. 115, no. 1-2, pp. 267-274, 1998.
W. B. Fu and T. F. Zeng, “A General Method for Determining Chemical Kinetics Parameters during Ignition of Coal Char Particles,” Combustion and Flame, vol. 88, no. 3-4, pp. 413-424, 1992.
S. Ünal, S. Pişkin, and S. Dinçer, “Autoignition Tendencies of Some Turkish Lignites,” Fuel, vol. 72, no. 9, pp. 1357-1359, 1993.
Q. M. Brewster, Thermal Radiative Transfer and Properties, New York: John Wiley & Sons, 1992.
A. B. Fuertes, E. Hampartsoumian, and A. Williams, “Direct Measurement of Ignition Temperatures of Pulverized Coal Particles,” Fuel, vol. 72, no. 9, pp. 1287-1291, September 1993.
P. Ghetti, L. Ricca, and L. Angelini, “Thermal Analysis of Biomass and Corresponding Pyrolysis Products,” Fuel, vol. 75, no. 5, pp. 565-573, April 1996.
W. B. Fu and E. Z. Zhang, “A Universal Correlation between the Heterogeneous Ignition Temperatures of Coal Char and Coals,” Combustion and Flame, vol. 90, no. 2, pp. 103-113, 1992.
T. Sonobe and N. Worasuwannarak, “Kinetic Analyses of Biomass Pyrolysis Using the Distributed Activation Energy Model,” Fuel, vol. 87, no. 3, pp. 414-421, March 2008.
D. Q. Tran and C. Rai, “A Kinetic Model for Pyrolysis of Douglas Fir Bark,” Fuel, vol. 57, no. 5, pp. 293-298, May 1978.
Published
How to Cite
Issue
Section
License
Copyright Notice
Submission of a manuscript implies: that the work described has not been published before that it is not under consideration for publication elsewhere; that if and when the manuscript is accepted for publication. Authors can retain copyright in their articles with no restrictions. Also, author can post the final, peer-reviewed manuscript version (postprint) to any repository or website.

Since Jan. 01, 2019, IJETI will publish new articles with Creative Commons Attribution Non-Commercial License, under Creative Commons Attribution Non-Commercial 4.0 International (CC BY-NC 4.0) License.
The Creative Commons Attribution Non-Commercial (CC-BY-NC) License permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.







.jpg)


