Carbon Dioxide Capture Performance of Mesostructured Adsorbent Impregnated with Polyethylenimine
DOI:
https://doi.org/10.46604/ijeti.2024.13298Keywords:
polyethylenimine, CO2 capture, kinetic model, mesoporous materialsAbstract
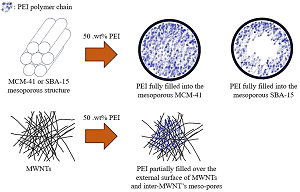
This study aims to investigate the CO2 uptake performance of mesostructured adsorbents, such as Mobil Composition of Matter No. 41 (MCM-41), Santa Barbara Amorphous-15 (SBA-15), and multi-walled carbon nanotubes (MWNTs), modified with polyethylenimine (PEI). Mesoporous materials are loaded with 50 wt.% PEI using a wet impregnation method. CO2 kinetic experiments of the PEI-modified adsorbents are conducted by a thermogravimetric method. The results reveal that the CO2 adsorption capacities of the PEI/MCM-41, PEI/SBA-15, and PEI/MWNTs composites are 2.02, 3.06, and 2.93 mmol/g, respectively, under 15% CO2 flow at 348 K. The lower CO2 adsorption capacity of PEI/MCM-41 (2.02 mmol/g) is attributed to its poor porosity. The PEI/MWNTs composite has the fastest CO2 adsorption and desorption kinetics at the same temperature, compared to other PEI-modified adsorbents. These results suggest that MWNTs might play a significant “separator” role in effectively dispersing the PEI molecular chains on the mesostructured adsorbent.
References
International Energy Agency, “Net Zero by 2050: A Roadmap for the Global Energy Sector,” IEA, Special Report, 2021.
M. Waseem, M. Al-Marzouqi, and N. Ghasem, “A Review of Catalytically Enhanced CO2-Rich Amine Solutions Regeneration,” Journal of Environmental Chemical Engineering, vol. 11, no. 4, article no. 110188, August 2023.
R. Neerup, V. E. Rasmussen, S. H. B. Vinjarapu, A. H. Larsen, M. Shi, C. Andersen, et al., “Solvent Degradation and Emissions from a CO2 Capture Pilot at a Waste-to-Energy Plant,” Journal of Environmental Chemical Engineering, vol. 11, no. 6, article no. 111411, December 2023.
X. Xu, C. Song, J. M. Andresen, B. G. Miller, and A. W. Scaroni, “Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture,” Energy & Fuels, vol. 16, no. 6, pp. 1463-1469, November 2002.
D. Panda, V. Kulkarni, and S. K. Singh, “Evaluation of Amine-Based Solid Adsorbents for Direct Air Capture: A Critical Review,” Reaction Chemistry & Engineering, vol. 8, no. 1, pp. 10-40, January 2023.
K. S. K. Reddy, A. M. Varghese, A. E. Ogungbenro, and G. N. Karanikolos, “Aminosilane-Modified Ordered Hierarchical Nanostructured Silica for Highly-Selective Carbon Dioxide Capture at Low Pressure,” ACS Applied Engineering Materials, vol. 1, no. 2, pp. 720-733, February 2023.
V. O. Viola, T. F. de Aquino, S. T. Estevam, B. Bonetti, H. G. Riella, C. Soares, et al., “Synthesis and Application of Two Types of Amine Sorbents Impregnated on Silica from Coal Fly Ash for CO2 Capture,” Results in Engineering, vol. 20, article no. 101596, December 2023.
M. Yang, S. Wang, and L. Xu, “Hydrophobic Functionalized Amine-Impregnated Resin for CO2 Capture in Humid Air,” Separation and Purification Technology, vol. 315, article no. 123606, June 2023.
B. Dziejarski, J. Serafin, K. Andersson, and R. Krzyżyńska, “CO2 Capture Materials: A Review of Current Trends and Future Challenges,” Materials Today Sustainability, vol. 24, article no. 100483, December 2023.
X. Xu, M. B. Myers, F. G. Versteeg, B. Pejcic, C. Heath, and C. D. Wood, “Direct Air Capture (DAC) of CO2 Using Polyethylenimine (PEI) “Snow”: A Scalable Strategy,” Chemical Communications, vol. 56, no. 52, pp. 7151-7154, July 2020.
T. V. R. Mohan, P. Sridhar, and P. Selvam, “Experimental and Modelling Studies of Carbon Dioxide Capture onto Pristine, Nitrogen-Doped, and Activated Ordered Mesoporous Carbons,” RSC Advances, vol. 13, no. 2, pp. 973-989, 2023.
H. Li, “CO2 Capture by Various Nanoparticles: Recent Development and Prospective,” Journal of Cleaner Production, vol. 414, article no. 137679, August 2023.
A. Heydari-Gorji, Y. Yang, and A. Sayari, “Effect of the Pore Length on CO2 Adsorption over Amine-Modified Mesoporous Silicas,” Energy & Fuels, vol. 25, no. 9, pp. 4206-4210, September 2011.
S. Loganathan, M. Tikmani, A. Mishra, and A. K. Ghoshal, “Amine Tethered Pore-Expanded MCM-41 for CO2 Capture: Experimental, Isotherm and Kinetic Modeling Studies,” Chemical Engineering Journal, vol. 303, pp. 89-99, November 2016.
S. Loganathan and A. K. Ghoshal, “Amine Tethered Pore-Expanded MCM-41: A Promising Adsorbent for CO2 Capture,” Chemical Engineering Journal, vol. 308, pp. 827-839, January 2017.
C. Dhoke, A. Zaabout, S. Cloete, and S. Amini, “Review on Reactor Configurations for Adsorption-Based CO2 Capture,” Industrial & Engineering Chemistry Research, vol. 60, no. 10, pp. 3779-3798, March 2021.
F. Raganati, F. Miccio, and P. Ammendola, “Adsorption of Carbon Dioxide for Post-Combustion Capture: A Review,” Energy & Fuels, vol. 35, no. 16, pp. 12845-12868, August 2021.
H. Lin, J. Lu, A. M. Abed, K. Nag, M. Fayed, A. Deifalla, et al., “Simulation of CO2 Capture from Natural Gas by Cyclic Pressure Swing Adsorption Process Using Activated Carbon,” Chemosphere, vol. 329, article no. 138583, July 2023.
A. S. Akdag, I. Durán, G. Gullu, and C. Pevida, “Performance of TSA and VSA Post-Combustion CO2 Capture Processes with a Biomass Waste-Based Adsorbent,” Journal of Environmental Chemical Engineering, vol. 10, no. 6, article no. 108759, December 2022.
Z. Wang, L. Liu, G. Tian, T. Ren, Z. Qi, and G. K. Li, “Effect of Isopropanol on CO2 Capture by Activated Carbon: Adsorption Performance and Regeneration Capacity,” Chemical Engineering Research and Design, vol. 196, pp. 632-641, August 2023.
M. Thommes, K. Kaneko, A. V. Neimark, J. P. Olivier, F. Rodriguez-Reinoso, J. Rouquerol, et al., “Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report),” Pure and Applied Chemistry, vol. 87, no. 9-10, pp. 1051-1069, October 2015.
H. Hu, T. Zhang, S. Yuan, and S. Tang, “Functionalization of Multi-Walled Carbon Nanotubes with Phenylenediamine for Enhanced CO2 Adsorption,” Adsorption, vol. 23, no. 1, pp. 73-85, January 2017.
Q. Liu, J. Shi, S. Zheng, M. Tao, Y. He, and Y. Shi, “Kinetics Studies of CO2 Adsorption/Desorption on Amine-Functionalized Multiwalled Carbon Nanotubes,” Industrial & Engineering Chemistry Research, vol. 53, no. 29, pp. 11677-11683, July 2014.
G. Bai, Y. Han, P. Du, Z. Fei, X. Chen, Z. Zhang, et al., “Polyethylenimine (PEI)-Impregnated Resin Adsorbent with High Efficiency and Capacity for CO2 Capture from Flue Gas,” New Journal of Chemistry, vol. 43, no. 46, pp. 18345-18354, December 2019.
X. Wang, L. Chen, and Q. Guo, “Development of Hybrid Amine-Functionalized MCM-41 Sorbents for CO2 Capture,” Chemical Engineering Journal, vol. 260, pp. 573-581, January 2015.
M. J. Al-Marri, Y. O. Kuti, M. Khraisheh, A. Kumar, and M. M. Khader, “ Kinetics of CO2 Adsorption/Desorption of Polyethyleneimine-Mesoporous Silica,” Chemical Engineering & Technology, vol. 40, no. 10, pp. 1802-1809, October 2017.
Y. Teng, Z. Liu, G. Xu, and K. Zhang, “Desorption Kinetics and Mechanisms of CO2 on Amine-Based Mesoporous Silica Materials,” Energies, vol. 10, no. 1, article no. 115, January 2017.
K. O. Yoro, M. K. Amosa, P. T. Sekoai, J. Mulopo, and M. O. Daramola, “Diffusion Mechanism and Effect of Mass Transfer Limitation during the Adsorption of CO2 by Polyaspartamide in a Packed-Bed Unit,” International Journal of Sustainable Engineering, vol. 13, no. 1, pp. 54-67, 2020.
Published
How to Cite
Issue
Section
License
Copyright (c) 2024 Chien-Hung Chen, Ching-Tsung Yu, Yu-Fei Chang

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright Notice
Submission of a manuscript implies: that the work described has not been published before that it is not under consideration for publication elsewhere; that if and when the manuscript is accepted for publication. Authors can retain copyright in their articles with no restrictions. Also, author can post the final, peer-reviewed manuscript version (postprint) to any repository or website.

Since Jan. 01, 2019, IJETI will publish new articles with Creative Commons Attribution Non-Commercial License, under Creative Commons Attribution Non-Commercial 4.0 International (CC BY-NC 4.0) License.
The Creative Commons Attribution Non-Commercial (CC-BY-NC) License permits use, distribution and reproduction in any medium, provided the original work is properly cited and is not used for commercial purposes.







.jpg)


